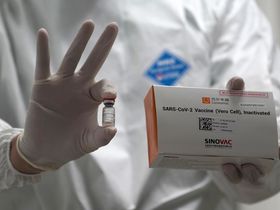
Sinovac Biotech Ltd. received regulatory approval from Chinese authorities for its coronavirus vaccine to be used by the general public in the country’s second such authorization.
The conditional approval was announced by the National Medical Products Administration on Saturday. Sinovac earlier said the protective efficacy of its vaccine, CoronaVac, met World Health Organization and China regulatory standards 14 days after the completion of two shots.
With the approval, the vaccine can be administered to the general population following one developed by state-owned China National Biotec Group Co. which got permission in December. The Chinese regulator had endorsed CoronaVac for emergency use in July.
China has fallen behind the U.S. and Europe in vaccinating its population. The more than 31.2 million doses administered since its official start date of Dec. 15 put it second only to the U.S., with its nearly 35 million shots. For a population of 1.4 billion, China has delivered a little more than two doses for every 100 people, compared to three in the European Union, 10 in the U.S. and nearly 60 in Israel, according to Bloomberg’s vaccine tracker.




Telegram中文下载 具有跨平台特性,可在手机、电脑和平板等设备上同步使用。